Chapter 55: Ischemia
Periventricular Leukomalacia
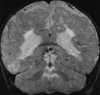
In developing or premature infants the vascular watershed zone is thought to be within the deep periventricular white matter. Thus global ischemia that occurs in the late second or early third trimester results in isolated periventricular leukomalacia (PVL).120 Symptoms range from mild irritability to severe encephalopathy; however, the most characteristic clinical presentation is spastic diplegia, a common form of cerebral palsy.120,123 On MRI, PVL typically appears as peritrigonal hyperintensities on T2-weighted images, ventriculomegaly, white matter volume loss, and atrophy of the corpus callosum (Figure 55-17). When white matter volume loss is pronounced, the ventricular margins follow the contour of the cortical gray matter and become wavy and irregular.
Dual Sinus and Venous Thrombosis
Cerebral venous occlusion can be the result of local or systemic factors. The local effects of infection, particularly sinusitis, trauma, or tumor (especially meningiomas), can occlude dural sinuses. Systemic disorders that produce coagulopathy (thrombocytosis, disseminated intravascular coagulation, systemic lupus erythematosus, contraceptives, and pregnancy) and those that result in stasis of venous return (dehydration, congestive heart failure, and polycythemia vera) can cause cerebral venous thrombosis. In children, infection and leukemia commonly predispose to dural venous thrombosis, whereas in adults, oral contraceptives, pregnancy, and systemic malignancy are frequently responsible.
Similar to arterial occlusive disease, the clinical presentation of cerebral venous thrombosis depends on both the extent and time course of the occlusive process, the location, and the venous collateral channels. Most commonly, nonspecific symptoms (e.g., hemiplegia, seizures, nuchal rigidity, or vomiting) occur, and the diagnosis is often overlooked. However, headache is almost always present, and hemiparesis, which frequently fluctuates in severity, is the most common deficit.72
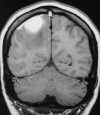
Usually, clots form in the venous sinuses and propagate to smaller vessels, which results in venous obstruction. This in turn creates an increase in proximal venous and capillary hydrostatic pressure, which may lead to only mild cerebral edema or ischemia or progress to frank hemorrhagic or nonhemorrhagic infarction. In addition, because major cerebral veins often drain more than one arterial territory, patterns of venous sinus infarcts differ from those of arterial infarction. Thus venous infarction and hemorrhage often cross the “watershed zone” to involve more than one vascular territory. For example, with superior sagittal sinus thrombosis, multiple, bilateral asymmetrical infarcts (which may be hemorrhagic) occur in the high cerebral convexity parasagittal regions, often involving both the ACA and MCA territories (Figure 55-18). Thrombosis of the straight sinus and its branches will damage the deep portions of the hemispheres and the basal ganglia. As described earlier, with venous occlusion there is an elevation of venous and capillary hydrostatic pressure that causes more extensive vasogenic edema than is usually seen with arterial infarction. Thus venous infarction is more likely to produce multifocal hemorrhagic infarcts and vasogenic edema and even lead to herniation.
On MRI, direct visualization of the thrombus within the sinus, as well as loss of a normal flow void, can be seen on routine spin-echo imaging.121 As expected, the signal intensity of the thrombus is time dependent. Absence of the flow void on spin-echo imaging can be the result of flow-related enhancement. Thus when dural sinus thrombosis is suspected, this finding should be confirmed in multiple planes on both T1- and T2-weighted images.121 In addition, MR venography has been shown to be an accurate, noninvasive method for the evaluation of dural sinus thrombosis. Good correlation has been shown between MR venography and digital subtraction angiography (DSA).86 MR venography has generally become the test of choice for the diagnosis of dural sinus thrombosis.128 PC MRA sequences should be used because of their sensitivity to slow flow (Figure 55-19). Care should be taken when TOF MRA is used because short-T1 methemoglobin can simulate slow flow.4 Whenever an abnormality is seen on the MIP image, both the individual source and standard conventional spin-echo images are necessary to confirm the finding. A positive diagnosis of the thrombosis can be made with certainty only when there is direct visualization of the thrombus because absence of a vein or sinus on MR venography could be due to the normal variation in venous anatomy.86
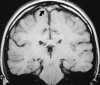 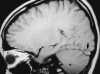 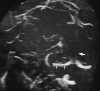 |
| Figure 55-19 A 21-year-old woman on oral birth control with severe headache. A, T1-weighted coronal image shows absence of the normal flow void within the superior sagittal sinus (arrow). B, T1-weighted sagittal image shows extension of the thrombus into the right transverse sinus (arrow). C, 2D TOF MR venogram shows absence of flow within the expected region of the superior sagittal sinus (large arrow) and irregularity of the right transverse sinus (small arrows) compatible with the diagnosis of dural venous thrombosis. There were no areas of signal alteration involving the brain to suggest ischemia or infarction. |
Migraine
Migraine affects 15% to 30% of the population and has a female predominance and a familial pattern of occurrence.118 Common migraine, the most common type of migraine headache, constitutes approximately 80% of all migraines. Common migraine is usually clinically recognized as a unilateral, throbbing, severe headache. The premonitory neurological disturbances either are completely absent or may be very vague and ill defined. Classic migraine is the best known and most easily recognized form of migraine, although it only constitutes about 10% of the migraine population. Classic migraine denotes migraine headache that is preceded by a prodrome of characteristic sensory, motor, or visual disturbances. These neurological disturbances generally last only a short time (up to about 30 min). When the neurological deficits last longer or result in permanent CNS deficit, the syndrome is referred to as a complicated migraine, a much less common entity.114,126
The cause of migraine headache remains incompletely understood. The current theory states that migraine has an initial vasoconstrictive phase that results in the characteristic aura and focal neurological symptoms. In patients with common migraine, presumably the vasoconstriction occurs; however, it is below the threshold necessary to produce obvious symptoms. The initial vasoconstriction is followed by vasodilatation resulting in the typical migraine headache that often throbs in unison with the pulse. Usually the vasoconstrictive symptoms are resolving or have resolved by the time the vasodilatory symptoms begin. Migraine sufferers also have an elevated frequency of cervical dissection.
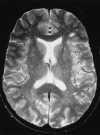
Foci of high intensity involving the white matter have been described in up to 46% of migraine sufferers.114 However, more recent studies report a much lower frequency of approximately 12%.97 These white matter abnormalities range from a few punctate foci of hyperintensity to more extensive, confluent involvement of both the periventricular and subcortical white matter (Figure 55-20).97,114 These abnormalities appear similar to lesions found in multiple sclerosis, arteriosclerotic disease, and vasculitis. Frank infarction can also be seen, especially with complicated migraine attacks (Figure 55-21).
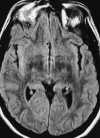 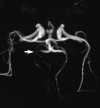 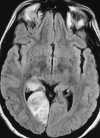  |
| Figure 55-21 Complicated migraine. A 32-year-old woman with a history of migraines presents with left hemianopsia and facial numbness. A and B, Turbo FLAIR axial and 3D TOF MRA, respectively, show only minimal signal alteration in the right occipital lobe but markedly diminished flow involving the right PCA (arrow) supplying this region. C and D, Subsequent turbo FLAIR axial and 3D TOF MRA obtained 6 days later show considerable signal alteration involving the right occipital lobe despite marked improvement in flow of the right PCA. These findings were presumed to represent development of edema caused by the initial vasospasm and subsequent vasodilatation. The patient's symptoms were resolved at this time. |
Hypertensive Encephalopathy
Hypertensive encephalopathy (HE) is an acute neurological syndrome that occurs in any patient with extreme hypertension. It is characterized by severe headache, nausea, vomiting, altered consciousness, and visual disturbance. The most common cause of HE is toxemia of pregnancy (eclampsia). Other less common, non–pregnancy-induced HE etiologies include thrombocytic thrombocytopenic purpura (TTP), chronic renal failure, and hemolytic-uremic syndrome. Eclampsia is a disorder of pregnancy occurring after the twentieth week of gestation and characterized by hypertension, proteinuria, edema, and seizures.71 Eclampsia causes 20% of maternal deaths in the United States, half of which are the result of cerebral complications.
Both the clinical presentation and cerebral pathology are very similar in patients with eclampsia and non–pregnancy-induced HE.108 Although the pathophysiology of HE is incompletely understood, recent theories have focused on cerebral autoregulatory system failure in the face of extremely high blood pressure. When the autoregulatory limits are exceeded, the cerebral arteriole may passively overdistend, which ultimately results in disturbance of the tight junctions between endothelial cells and extravasation of fluid and proteins across an altered BBB with resultant vasogenic edema (Figure 55-22).108 MR studies have shown that two different patterns of brain involvement—the posterior cerebral circulation pattern and the basal ganglia–deep white matter pattern— are usually seen.108,111 Involvement of the posterior circulation is the most common pattern. The lesions tend to occur in the cortex and subcortical white matter of primarily the occipital and posterior parietal lobes. These foci are generally bilateral and symmetrical and follow the gyri in a serpentine manner. The lesions that occur in the basal ganglia and deep white matter are often bilateral; however, they have a more globular or linear appearance (as opposed to serpentine) and lack symmetry. Surprisingly, most of these lesions show complete resolution on follow-up MR studies (Figure 55-23).108 Although autopsy findings in fatal cases of eclampsia show multiple areas of microhemorrhage and microinfarct, only rarely have large areas of hemorrhage or infarction been reported on imaging studies and to our knowledge have never been reported in non–pregnancy-induced HE.111 This may be because the lesions are below the limit of resolution of current MR scanners or because the patients who are able to undergo an MR examination have experienced less severe clinical courses. The reason behind the predilection for the lesions to occur in these two areas of the brain is unknown. One hypothesis states that the lesions occur in the posterior circulation as a result of the relatively poor sympathetic innervation of the vertebrobasilar system (as compared with the anterior circulation), which protects the brain from pronounced increases in blood pressure.111
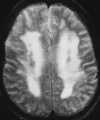 |
| Figure 55-22 A 59-year-old man with hypertensive crisis and altered mental status. T2-weighted axial image shows diffuse high signal intensity throughout the centrum semiovale. |
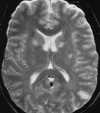 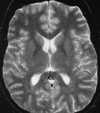 |
| Figure 55-23 Eclampsia. A, T2-weighted axial image shows multiple foci of signal alteration involving both the basal ganglia and posterior left parietal lobe. B, These findings resolved completely on follow-up examination, obtained 6 months later. |
~Previous ~ Next ~
~ Back to Chapter Index~
~ Back to Magnetic Resonance Imaging main page ~