Chapter 55: Ischemia
Clinical Entities Associated With Cerebral Ischemia
Atherosclerosis
Atherosclerosis is the leading cause of stroke. Compared with atherosclerotic coronary artery disease, cerebrovascular disease usually becomes symptomatic later in life and has only a slight male predominance.130 Multiple risk factors, including heredity, hypertension, diabetes mellitus, smoking, previous strokes, and cardiac disease, appear to be involved.133 Although the pathophysiology of atherosclerosis is not completely understood, the major theory suggests that blood-borne lipids, especially cholesterol esters, interact with vascular injury and repair mechanisms to form fibrofatty plaques within the vessel intima. These atheromata, located at the bifurcation and curvature of large arteries, are composed of a lipid core (mostly cholesterols compounded to proteins), a proliferation of smooth muscle cells, and a fibrous cap. These plaques frequently narrow the vessel lumen and undergo ulceration, hemorrhage, and calcification, thereby commonly serving as a nidus for thrombosis and a source of embolization. The common carotid artery bifurcation tends to be the most severely affected site. Although total blockage of the system can be asymptomatic (because three other vessels contribute to the common circle of Willis), preocclusive disease in this location commonly produces clinical effects ranging from TIA to embolic or, less commonly, hemodynamic stroke. The primary occlusion of large intracerebral arteries by atherosclerosis is uncommon.68,74,133
Traditionally, the sudden onset of ischemic symptoms was thought to be pathognomonic of thromboembolism, whereas a stuttering, more slowly developing course was believed to reflect primary vascular narrowing with hemodynamic compromise. It is now known that these presentations are not mutually exclusive because multiple sequential emboli or lysis or movement of a single intracerebral clot can result in an evolution of symptoms. However, with the thromboembolic events, symptoms generally occur suddenly and typically reflect ischemia of the MCA territory. Many patients who suffer a thromboembolic stroke have no history of antecedent TIAs. On the other hand, hemodynamic infarction often presents as a cortical-subcortical watershed insult, which evolves less rapidly, has milder symptoms, and is more likely to be preceded by TIAs.100
The MR appearance of thromboembolic infarction is largely dependent on the vascular distribution of the occluded vessel and its collateral support. Thus a good working knowledge of the major vascular territories is essential for appropriate interpretation of the MR findings. (See Chapter 50 for a detailed discussion of the intracranial vascular anatomy.) Bear in mind, however, that the major vascular territories vary from individual to individual and do not have fixed boundaries and that the location of these boundaries is governed by the underlying hemodynamic conditions.124 Infarction usually encompasses both white and gray matter but predominates in the latter, often has a triangular or rectangular shape, and is frequently sharply marginated. By comparison, neoplasia and inflammation tend to be located within the white matter or at the gray-white junction and extend into the cortex secondarily. In addition, these processes are not restricted by the boundaries of a vascular territory. Nonischemic lesions also are more likely to be round or amorphous, to be poorly marginated, and to track edema in white matter in a “pseudopod” configuration, producing greater mass effect than acute infarction. As expected, the characteristic acute onset and temporal evolution of infarction are not seen with neoplasia or inflammation.48,101
Each of the three major cerebral arteries—the anterior (ACA), middle (MCA), and posterior (PCA)—give rise to two types of branches—the deep ganglionic and cortical branches. The cortical branches supply the cerebral cortex and underlying white matter of the cerebral hemispheres. The peripheral cortical branches have substantial anastomoses between the three major cerebral arteries. The ganglionic branches arise from the circle of Willis, and the proximal portions of the major cerebral arteries penetrate the substance of the brain to supply the deep structures. These deep ganglionic branches are commonly referred to as end vessels because of their insufficient collaterization.35 There are also anastomoses, albeit poor ones, between the deep ganglionic and cortical circulation.
The majority of strokes occur in the MCA territory (75%) followed by the PCA territory, probably reflecting the relative distribution of total brain blood supply. The MR appearance of MCA territory infarction varies with the occlusion site. Complete MCA occlusion affects the anterior two thirds of the internal capsule, portions of the globus pallidus and putamen, and much of the caudate nucleus. The cortical branches supply much of the cerebral cortex and deep white matter, as well as a large portion of the posterior limb of the internal capsule (Figure 55-11). Classically a patient with complete MCA occlusion has contralateral hemiplegia and sensory loss (most pronounced in the upper extremity and face), homonymous hemianopsia, and conjugate eye deviation to the side of the lesion. Furthermore, involvement of the dominant hemisphere can cause global aphasia. However, when occlusion of the MCA occurs distal to its M1 segment, the lentiform nucleus and the anterior two thirds of the internal capsule and caudate nucleus are spared.
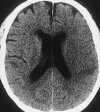 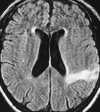 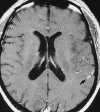 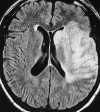 |
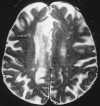
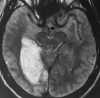
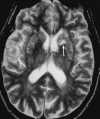
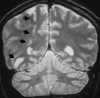
| Figure 55-11 Progressive left MCA infarct. A, Initial CT scan shows diffuse, vague low attenuation involving the left parietal lobe with loss of the gray-white matter distinction and sulcal effacement compatible with an acute left MCA territorial infarct. B, Turbo FLAIR axial image obtained 6 hours later shows similar findings involving the left MCA distribution; however, a bandlike area of infarction involving the posterior aspect of the left parietal lobe is more apparent. C, The corresponding enhanced T1-weighted axial image is remarkable for a left MCA vascular enhancement and a small focus of petechial hemorrhage (arrow). D, Turbo FLAIR axial image obtained 1 day later shows the extent of the left MCA territory infarct. |
The ACA supplies the medial hemispheric surfaces and the anterior two thirds of the parasagittal cerebral convexities (Figure 55-12). Anomalies of the ACA, which include unpaired arteries and instances in which branches are given off to the contralateral hemisphere, are seen in approximately 25% of patients.35 The recurrent artery of Heubner is a lenticulostriate branch that typically arises from the proximal A2 or A1 segment. It supplies the anteromedial part of the head of the caudate nucleus and adjacent portions of the internal capsule. Occlusion of this branch shows a classic wedge-shaped infarct lateral to the frontal horn of the lateral ventricle. The ACA usually possesses good collateral flow through its paired contralateral vessel, and thus isolated ACA infarcts are rare (0.6% of all infarcts).96 When occlusion of the ACA occurs, it generally produces a contralateral hemiplegia that is greatest in the lower extremity (see Chapter 55). When both ACAs arise from the common trunk and this occludes, paraplegia, lower extremity sensory loss, and incontinence are present and can mimic a spinal cord lesion. Obstruction of both ACAs is also associated with mental compromise (which can be severe), apraxia, and primitive grasp and suck reflexes.
Most commonly, the PCAs are formed by the bifurcation of the basilar artery. Generally the PCA supplies the inferior aspect of the temporal lobe, the posterior one third of the cerebral convexity, and the occipital lobe (Figure 55-13). Its ganglionic branches supply the caudal half of the thalamus and much of the midbrain. The calcarine artery is a branch of major importance because it supplies the primary visual cortex. Thus occlusion of the PCA or calcarine artery produces a contralateral homonymous hemianopsia. Vascular occlusive disease is common at the basilar tip and can result in bilateral PCA territorial infarcts.
The other principal branches of the basilar artery are the superior cerebellar artery (SCA), the anterior inferior cerebellar artery (AICA), and numerous brainstem perforators. The posterior inferior cerebellar artery (PICA) arises from the distal vertebral artery. With the exclusion of the most rostral portion of the cerebral peduncles, virtually the entire blood supply of the mesencephalon, pons, cerebellum, and medulla is supplied by the vertebrobasilar system. Although the distal anastomoses between the SCA, AICA, and PICA help to limit infarction in the cerebellum, the collateral supply to the brainstem is generally poor. Many patients with cerebellar and brainstem infarction are neurologically devastated. In addition, the volume of infarction can seem quite insignificant compared with the amount of resultant neurological damage. Because of the beam-hardening artifact, CT is quite limited in the detection of posterior fossa infarcts, which are frequently seen with MRI (Figure 55-3).
Small-Vessel Arterial Occlusive Disease
Lacunar infarcts are often multiple and account for approximately 16% to 25% of all strokes.102 Lacunes are thought to be the result of narrowing or occlusion of the small perforating branches of the MCA, PCA, basilar artery, and choroidal artery.19 Although the specific etiology remains debatable, lacunes have been attributed to microatheroma, lipohyalinosis, and emboli.25,46,129 As previously stated, the deep structures of the brain supplied by these ganglionic vessels are more susceptible to infarction as a result of deficient collateral interconnections. On MRI, lacunar infarcts are seen as small ovoid or slitlike foci of hyperintensity on proton density– and T2-weighted images, involving the basal ganglia, thalamus, internal capsule, and brainstem (Figure 55-14).19,25 Lacunar infarcts can enhance in the acute or subacute setting.90,102 With time, these small foci become hypointense to brain on T1-weighted images and can be difficult to differentiate from dilated perivascular spaces. However, the Virchow-Robin (VR) (perivascular) space are isointense to CSF on all pulse sequences and are not bright on proton density–weighted or FLAIR images. They are most commonly found adjacent to the anterior commissure, surrounding the lenticulostriate arteries.59,60.70
Both focal and confluent areas of signal alteration can be seen in the deep hemispheric white matter on T2-weighted images in asymptomatic elderly individuals, with a reported incidence ranging from 30%18 to as high as 80%59 (Figure 55-7). Hypertension and increasing age are significantly associated with the deep white matter lesions.8,9,18,23,134 It is thought that these regions are more vulnerable to a significant drop in blood pressure because they are supplied by long perforating arteries. The lengthy course of these vessels and their relative sparsity make them more vulnerable than surface arteries to aging and hypertensive vascular changes, which also affects their ability to effectively autoregulate. Histologically these hyperintensities represent a spectrum of lesions ranging from atrophic perivascular demyelination to gliosis to frank infarction.8,59,81 These lesions are hyperintense to brain parenchyma on proton density– and T2-weighted images and generally are not seen on T1-weighted images. The histological spectrum of these lesions cannot be reliably differentiated on a T2-weighted image. It has been shown, however, that the large, predominately subcortical lesions tend to represent true infarcts (i.e., necrosis, axonal loss, and cavitation), with surrounding gliosis.81 Such areas of frank infarction have CSF intensity on T1-weighted images as a result of cavitation.59
Ependymitis granularis can have a similar MR appearance to the noninfarcted, small vessel ischemic changes. Ependymitis granularis is present adjacent to the anterolateral angles of the frontal horns and perioccipitally. These focal hyperintensities are caused by breakdown of the ependymal lining resulting in increased periependymal extracellular fluid. The subependymal periventricular hyperintensity is considered a normal finding in patients over the age of 40 and becomes progressively thicker with increasing age.116
If deep white matter changes are accompanied by dementia in hypertensive patients, a diagnosis of subcortical atherosclerotic encephalopathy (SAE) is often applied. This term, first described by Binswanger in 1894,18,88 reflects the most severe manifestation of a spectrum of ischemic insults to the deep white matter and is a form of multi-infarct dementia (MID). Such patients generally experience stepwise, accumulative episodes of neurological deficits. SAE classically has additional features of pseudobulbar palsy and prominent motor signs.88 True MID is characterized on MRI by numerous cortical and subcortical infarcts, diffuse cerebral atrophy, and a higher prevalence of deep white matter lesions when compared with age-matched controls.51
Hypoxic-Ischemic Encephalopathy
Hypoxic-ischemic encephalopathy is caused by an inadequate amount of oxygen delivered to the brain, which may be the result of hypoxia (an insufficient oxygen content in the blood) or ischemia (a decrease in vascular perfusion). Hypoxia may be the result of conditions such as central respiratory failure, profound neonatal asphyxia, near drowning, or carbon monoxide inhalation. Hypoperfusion, on the other hand, may be secondary to cardiac dysrhythmia or arrest, hemorrhage, or severe prolonged hypotension. Hypoxia and hypoperfusion go hand in hand because respiratory failure eventually leads to cardiac failure, and conversely, cessation of adequate cardiac output often results in respiratory arrest, most likely secondary to brainstem ischemia. Despite a moderate reduction in perfusion pressure, cerebral blood flow often can be maintained as a result of autoregulatory vasodilatation. However, when the autoregulatory process fails, the result is global hypoperfusion.
Global ischemia or hypoxia often leads to cerebral injury in a “watershed” distribution that is often most pronounced at the parietooccipital junction, which is the convergence of the ACA, MCA, and PCA territories117 (Figure 55-15). The third, fifth, and sixth cortical layers are more sensitive to hypoxia and ischemia, and generalized cortical laminar necrosis, which is often associated with petechial hemorrhage, can result.117 Furthermore, the basal ganglia are prone to hypoxic insult, particularly in the head and body of the caudate nuclei, the putamen, and the thalamus (Figure 55-16). In the cerebellum, Purkinje's cells often demonstrate damage after hypoxia, and in the brainstem the reticular zones are especially sensitive to oxygen deprivation. Thus a diffuse or focal pattern of injury results in the more hypoxic-ischemic–sensitive structures of the brain, which often can lead to diffuse cerebral edema and death of the patient.
The late third-trimester, perinatal, or postnatal infant with a hypoxic-ischemic insult suffers injury to the deep gray matter nuclei and cortex and the subcortical white matter.120,123 Severe generalized cerebral edema, which may be concealed by the considerably higher overall water content and lack of myelination in the neonatal brain, can ensue, resulting in a nearly anatomically featureless organ.91
~ Previous ~ Next ~
~ Back to Chapter Index~
~ Back to Magnetic Resonance Imaging main page ~